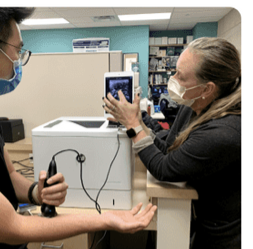
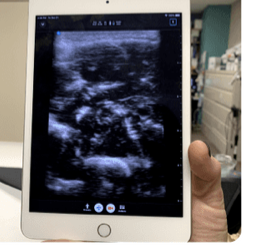
Designing a remote patient monitoring device: from clinical need to market launch
for University of Pennsylvania Health System
2022
The problem
80% of chronic heart failure patients develop pulmonary edema, a condition in which the patient's lungs fill up with fluid due to pressure differences that arise when the heart is beating abnormally. These 6.5M patients have no accurate way to monitor pulmonary edema at home, leading to a 28% readmission rate costing $33B annually.
My team developed Ultra@Home to address this critical gap in heart failure management. The project team consisted of a researcher, a UX designer, and three bioengineers including myself.
My role focused on the commercialization strategy: validating clinical need through physician interviews, defining competitive positioning, and building a go-to-market plan with regulatory and reimbursement pathways.
Designing Ultra@Home was not an exercise in finding the coolest technology — it was a disciplined, research-first process driven by user needs. We moved from need statement to market strategy through every stage: gap analysis, prior art review, patient journey mapping, stakeholder interviews, ideation, prototyping, and usability testing, documenting each step in a design history file.
Customer and stakeholder research
We started with primary and secondary market research to understand the root cause of the high readmission rates and lower quality of life for patients with pulmonary edema. We interviewed 10 stakeholders: surgeons, pulmonary medicine physicians, radiologists, and sonographers spanning the University of Pennsylvania Health System.
Key research insights:
- 6.5M heart failure patients in the U.S., 80% experience pulmonary edema
- $33B annual cost burden, 28% readmission rate, $34K per hospitalization
- Patients are prescribed diuretics titrated to their weight to manage blood pressure
- Existing at-home monitoring relies on unreliable weight changes
- Gold standard (lung ultrasound) requires hospital visits
- Absence of real-time monitoring = unreliable patient self-reporting
- Patients delay seeking care until symptoms are severe, requiring hospitalization
- Early detection enables intervention before disease progression and hospital admission
Informed by our research, I mapped the typical cycle of care for a pulmonary edema patient.
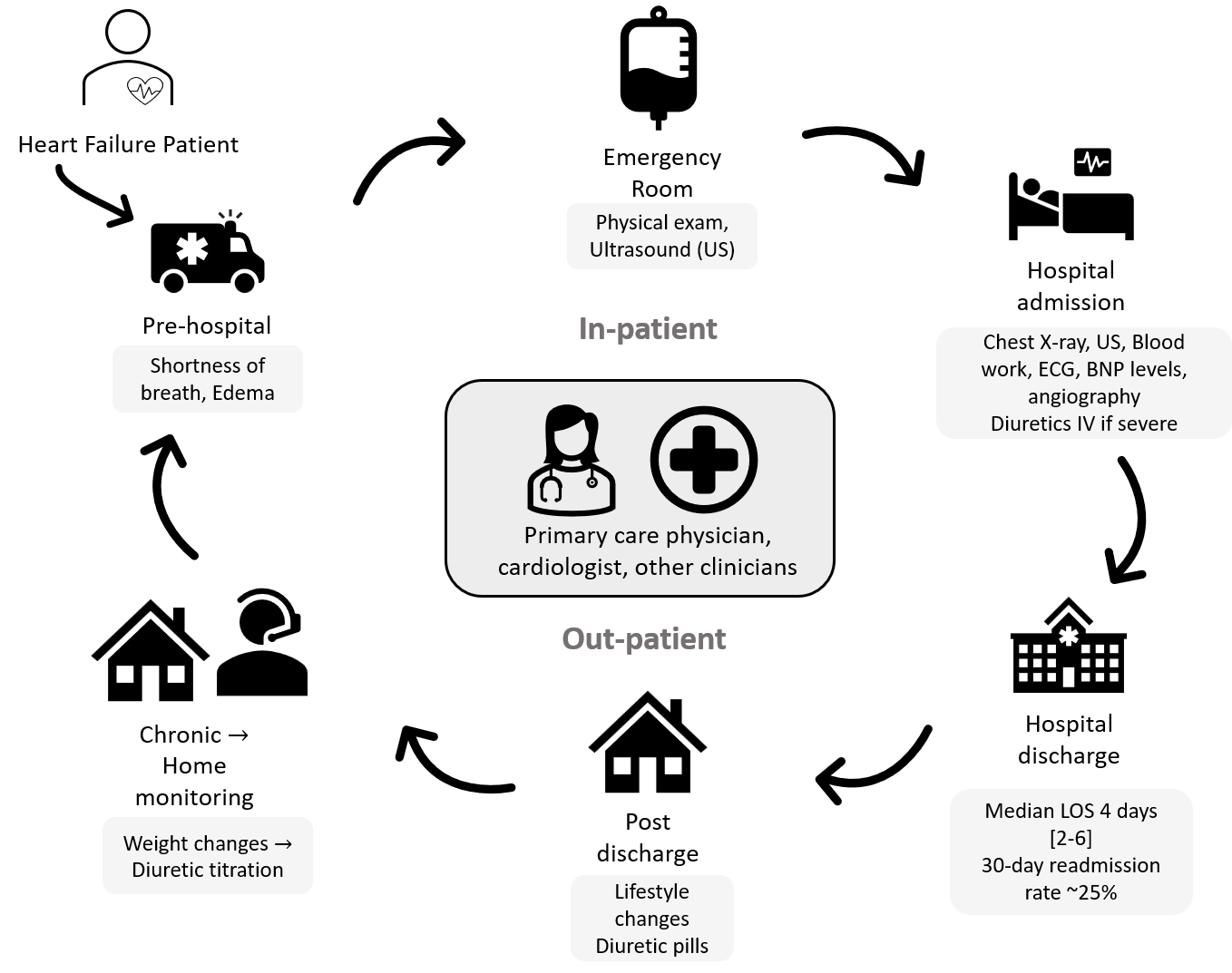
Stakeholder analysis
Before we designed a remote patient monitoring system, we listed the stakeholders, and our value proposition to each.
| Stakeholder | Role | Value Proposition | Barriers |
|---|---|---|---|
| Patients (mild) | End users | Convenience, safety, reduced office visits for routine monitoring, improved QoL | Digital literacy, training, compliance |
| Patients (severe) and caregivers | End users | Reduction in ER readmissions, safety | Digital literacy, training, compliance, physical ability |
| Physicians | Early evangelists | Increased throughput, reduced readmissions, better patient data | Workflow integration, image interpretation at scale |
| Hospitals | Economic buyers | Lower readmission penalties, reduced length of stay, cost savings | Upfront investment, training resources |
| Payers (CMS/Insurance) | Economic buyers | $20M+ savings from reduced hospitalizations | Reimbursement precedent, clinical validation |
Gap analysis
We conducted a gap analysis on the existing methods of patient monitoring.
| Diagnostic Method | Non-invasive | Cost | Time Required | Accuracy (EVLW) | Specificity to Pulmonary Edema | At-home Friendly |
|---|---|---|---|---|---|---|
| Chest auscultation | +++ | +++ | +++ | - - | - - | ++ |
| Chest X-ray | ++ | - - | + | + | + | - - - |
| Lung ultrasound | ++ | - | + | +++ | +++ | - |
| TPTD | - - - | - - - | +++ | +++ | +++ | - - - |
| Weight monitoring | +++ | +++ | +++ | - - - | - - - | +++ |
+ indicates an advantage; - indicates a disadvantage. Number of symbols indicates relative degree. Abbreviations: TPTD, transpulmonary thermodilution; EVLW, extravascular lung water (a measure of fluid accumulation in the lungs).
We now had enough insights to formulate a need statement.
Need statement
A need for a standardized and specific home monitoring system for pulmonary edema in heart failure patients, that improves quality of life of HF patients and reduces hospital readmission rates.
Ideation and prototype
We defined 5 critical user needs the solution should address and a corresponding design input that would satisfy each need. Our team ideated different solutions to solve for our need statement across modalities, technologies and intervention methods.
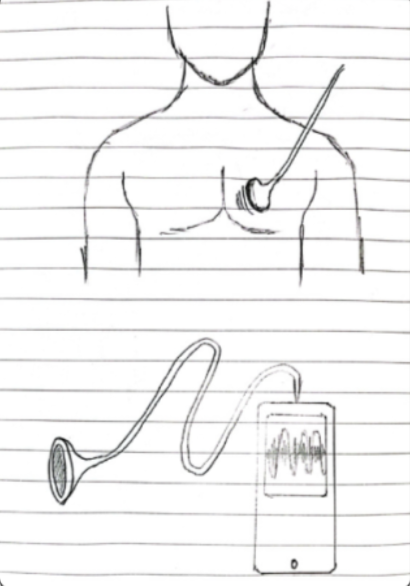
Stethoscope with automatic lung sound classifier
At-home portable ultrasound
Chest strap with bi-directional lung ultrasound probes
Wireless spirometer with O₂ & CO₂ partial pressure sensors
Pulse oximeter and finger prick blood test
We then used a decision matrix that incorporated weighted user needs to arrive at the Ultra@Home kit.
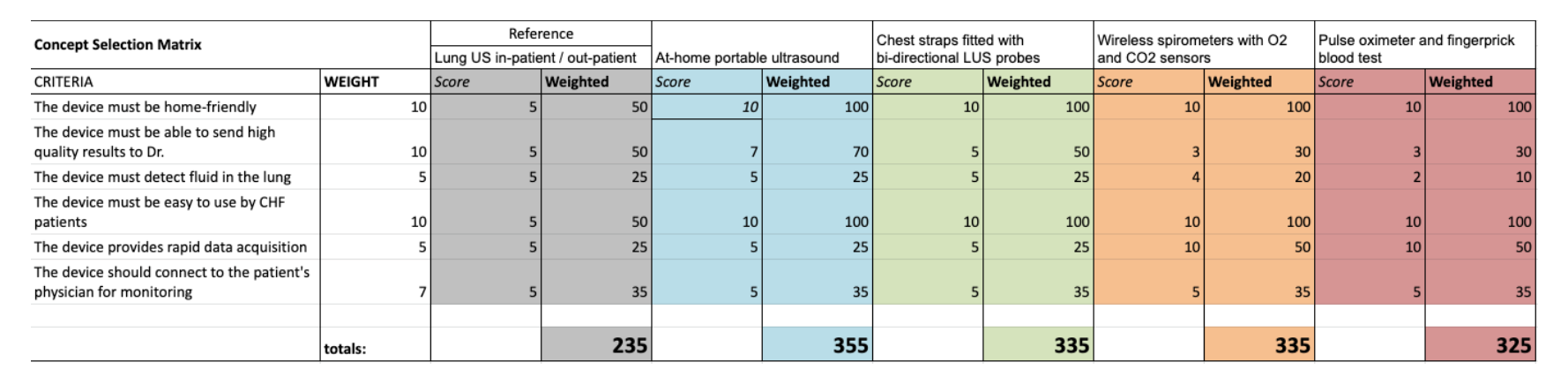
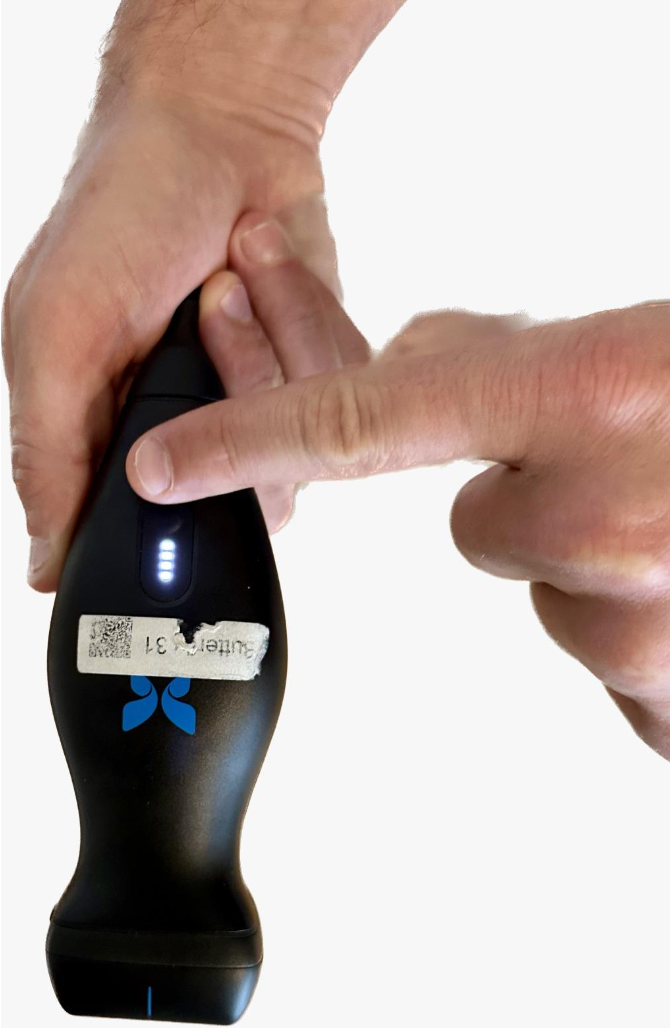
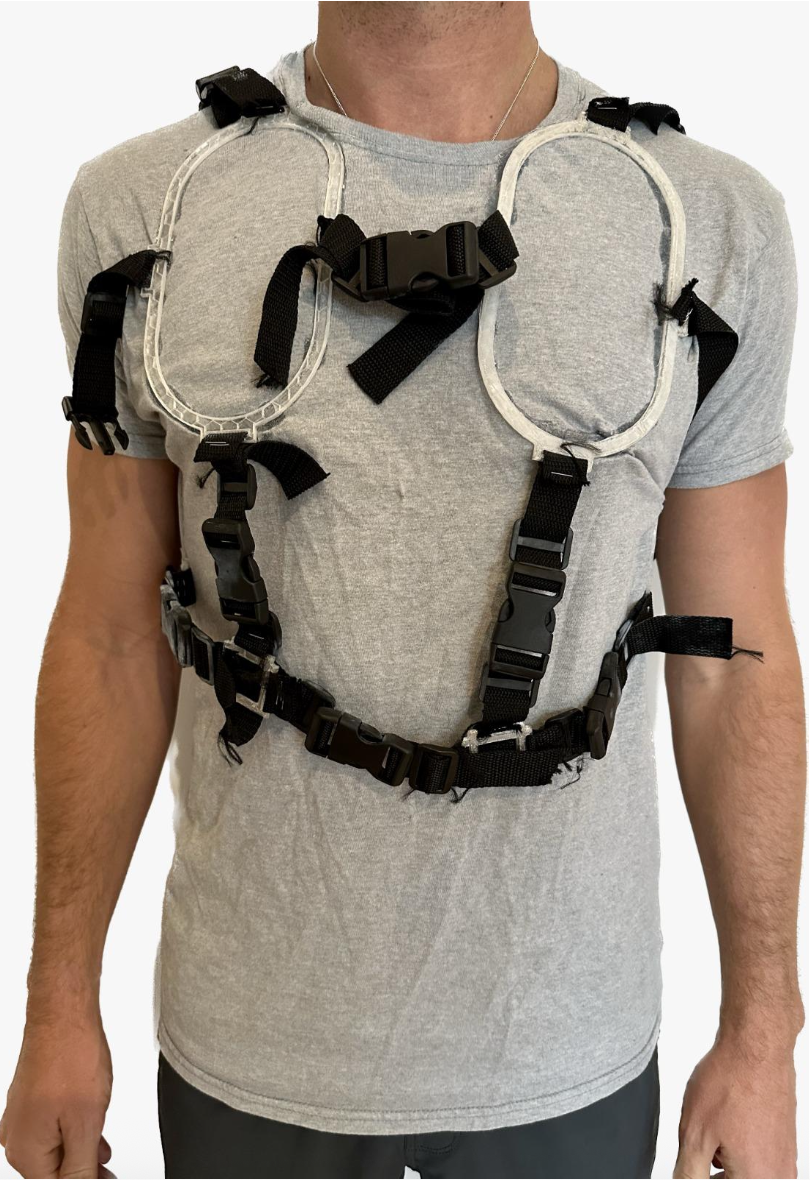
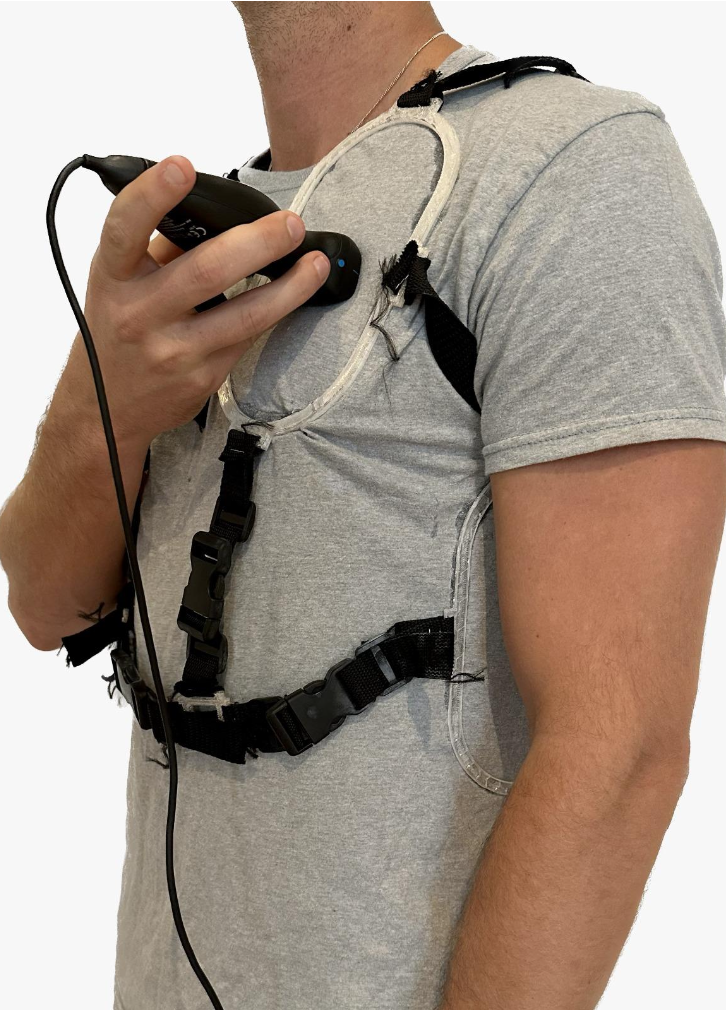
We built a prototype consisting of:
- A home-friendly portable ultrasound probe
- A vest that would indicate the specific locations of the chest at which the probe is to be placed
- A digital solution in the form of a smartphone application which would guide patients on the use of the device
Physical product
Digital product
Collaborating with the UX Designer on the team, I learnt how to build high-fidelity wireframes in Figma.
Onboarding
- Introduces the patient to the Ultra@Home care model in simple, accessible language
- Explains what is required of them and sets expectations for the monitoring process
- Connects them to their care team from the start
New Scan
- Guides the patient step-by-step through performing the lung ultrasound at home
- Uses visual indicators to show exact probe placement on the chest
- Captures and logs scan results for the care team to review
Care Coordination
- In-app messaging flow connecting patients directly to their care team
- Provides access to FAQs and a running scan log for ongoing reference and tracking
- Keeps patients engaged and supported between clinical visits
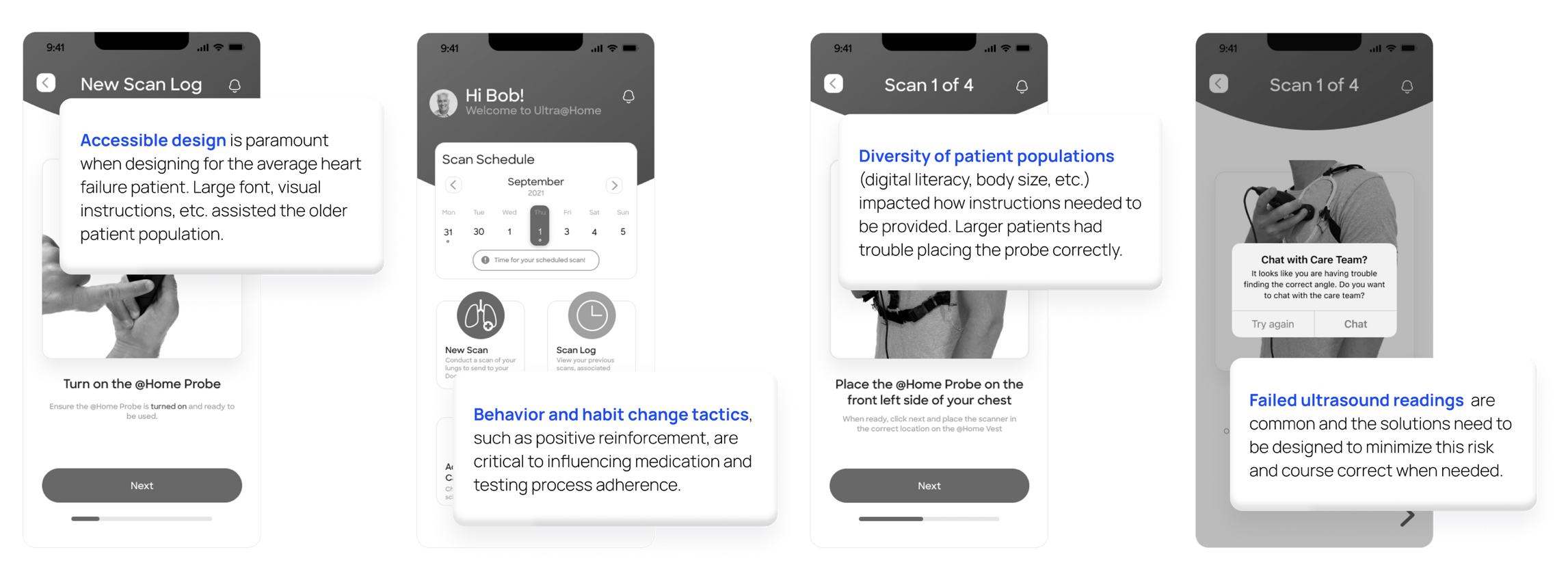
Usability testing
Physicians and patients followed the workflow with targeted questions to help us identify opportunities for improvement with both the digital and physical components. I also tested the digital prototype with a personal connection who was a Product Manager for a remote patient monitoring product at Roche.
Key learnings that we incorporated were:
Go-to-market strategy
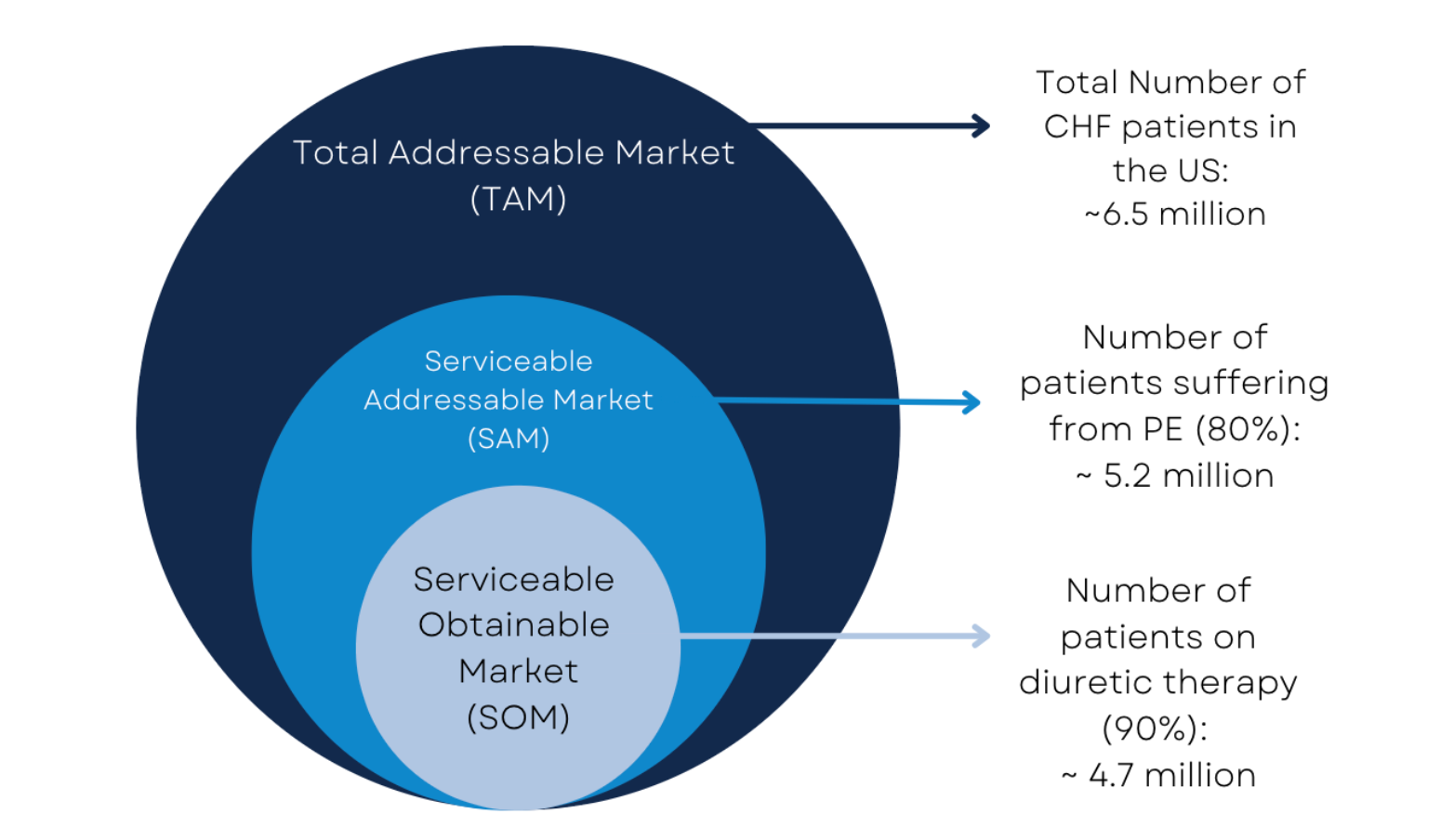
Market sizing
We identified a total addressable market of 6.5 million patients in the US, with 4.7 million currently in the critical treatment gap with the urgent unmet need.
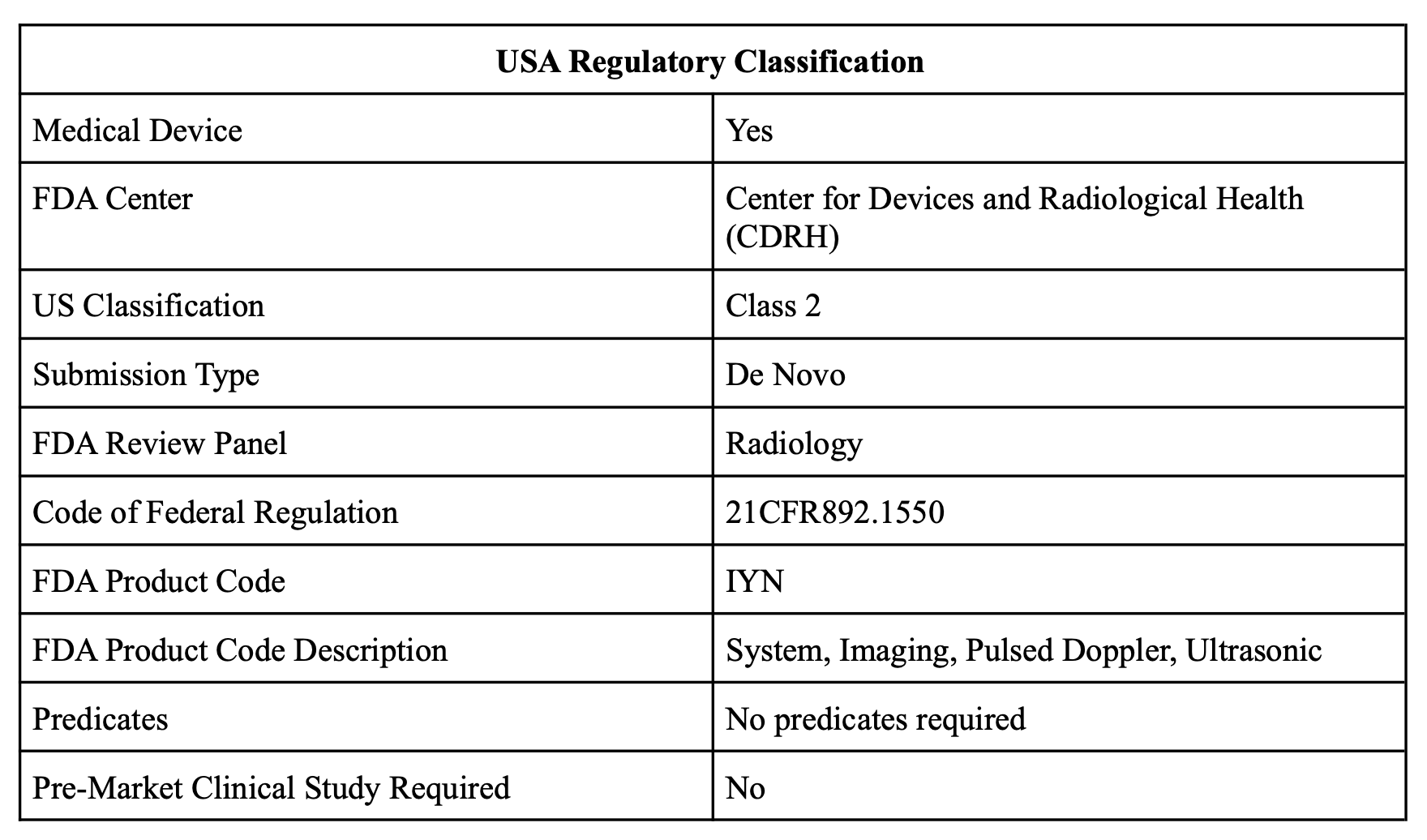
Regulatory pathway
We identified the FDA device class and approval pathway to pursue, based on patient risks and predicates in the market. This is a necessary step in commercialization.
Business model and pricing
Revenue Model
- Hardware: $2,400 one-time fee (ultrasound probe + vest)
- Software: $420/year subscription (app + cloud storage + physician portal)
- Total Year 1: $2,820 per patient
- Revenue projected to hit $300M+ by Year 5
Pricing Rationale
- Benchmarked against Butterfly iQ+ portable ultrasound ($2,400 device + annual subscription)
- Value-based: Saves $34K per prevented hospitalization → 12:1 ROI for hospitals
- Freemium first month to drive adoption and training completion
Business Model: B2B2C Hospital Rental
- Hospitals purchase devices
- Rent to prescribed HF patients
- Reimbursement via CMS/insurance (CPT code 76604: Diagnostic Ultrasound Procedures of the Chest)
Key takeaways
- Healthcare requires multi-stakeholder alignment. Clinical efficacy or cool technology alone doesn't drive adoption — you need simultaneous value for patients (UX), physicians (workflow), hospitals (economics), and payers (outcomes).
- Be obsessed with a problem, not married to a particular solution. Listening to users, building a hypothesis, and testing if you satisfy their needs >> building something cool under untested assumptions.
- Patient compliance is a critical factor in adoption. Simple, life-saving tasks get shockingly low compliance rates. Looking back in 2025, I think an even simpler solution - not necessarily the most accurate or sophisticated, but something that garners higher patient compliance - might be a better solution. For example, an IoT weighing scale that drops daily weights to the doctor's office - something like Wanda Health (launched in 2025).